Palladium Membranes
A palladium membrane is a high-performance metal membrane engineered to selectively permit hydrogen gas to permeate through
Hydrogen molecules diffuse into the palladium, become ionized, and are released back into a gaseous state - enabling high-purity hydrogen separation

Operating Principle
- Feed gas containing small molecules is introduced to one side of the palladium membrane
- Hydrogen molecules are ionized on the palladium surface and diffuse through the metal
- Inside the membrane, hydrogen ions recombine into gaseous hydrogen and are discharged on the opposite side
- The membrane selectively blocks all other gases, enabling hydrogen-only permeation
Key Features of Palladium Membranes
| Features | Description |
|---|---|
| Hydrogen Selectivity | Palladium selectively permeates only hydrogen, blocking other gases |
| Heat Resistance | Maintains excellent performance even in high-temperature environments, up to 300°C to 500°C |
| Chemical Resistance | Exhibits outstanding chemical resistance, even under oxidizing conditions |
| Hydrogen Permeability | Offers fast and efficient hydrogen diffusion properties |
| High-Purity Hydrogen Purification | Delivers ultra-high purity hydrogen through precise separation mechanisms |
Main Applications
| Field | Use Case |
|---|---|
| Hydrogen Purification | High-purity separation of hydrogen for industrial and laboratory gases |
| Fuel Cells | Purification systems for ultra-pure hydrogen supply |
| Semiconductors | Used in processes requiring extremely high hydrogen purity |
| Petrochemicals | Hydrogen separation during the FCC (Fluid Catalytic Cracking) process |
| Chemical/Bio | Hydrogen purification for analytical and precision manufacturing environments |
Hydrogen Purifier
- Pure H2 : ≥4N, Highly pure H2 : ≥5N, Ultra pure H2 : ≥6N, Ultra high H2 : ≥7N
- Raw hydrogen
- Catalytic Reforming (coal, natural gas, methanol, ethanol, dimethyl ether, gasoline, etc.)
- Chemical Decomposition (ammonia, ammonia borane, sodium borohydride, hydrides, formic acid, aluminum + base, silicon + base, etc.)
- Electrolyzed Water (alkaline solution, pure water)
- Bio-Hydrogen Production (microbial fermentation, photosynthetic algae, biomass gasification, etc.)
- Various By-product Hydrogen Sources (chlor-alkali, refining, petrochemical, coke, coal chemical, waste treatment, etc.)
- H₂ Capacity: Varies depending on membrane surface area and operating conditions

- H₂ production: 0.05~1000 m3/h
- H₂ purity: 99.999~99.9999999%
- Feed pressure: 0.5~50 bar
Hydrogen Purifier for CVD
CVD hydrogen purifiers based on palladium membranes deliver hydrogen with 8N–12N purity, ideal for CVD processes in semiconductor chip and TFT-LCD production

CVD (Chemical Vapor Deposition) - a technique used to deposit solid thin films on substrates via chemical reactions in high-temperature environments
Widely used in MPCVD processes for the production of high-quality synthetic diamonds with enhanced clarity and color
(Especially suited for semiconductor and diamond manufacturing applications)

MPCVD (Microwave Plasma CVD) - a technology primarily used for diamond thin film growth and nanomaterial synthesis
Hydrogen/Helium Separator
- Hydrogen and helium, due to their small molecular sizes and similar physical properties, are challenging to separate
- However, by combining palladium membranes with Pressure Swing Adsorption (PSA), 99.999% purity for each gas can be achieved

Main Applications
| Field | Use Case |
|---|---|
| Semiconductor Manufacturing | Situations requiring high-purity hydrogen and helium |
| Bioanalysis | Processes demanding precise gas separation |
| Aerospace Industry | Helium applications in rocket fuel and cryogenic cooling |
| Energy Industry | Used in natural gas and biogas purification |
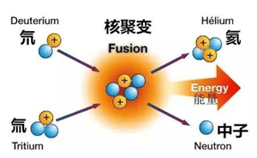
H₂ (Hydrogen) / D₂ (Deuterium) / T₂ (Tritium) Separator
- The separation selectivity of Pd membranes among H₂, D₂, and T₂ is approximately 1.3–2; however, isotopic molecules such as HD, HT, and DT may be generated
- T₂ is primarily produced through nuclear reactions between thermal neutrons and ⁶Li in reactors, and the resulting T₂ and He can be separated using palladium membranes
- T₂ and D₂ fusion reactions have potential applications as sources of clean nuclear energy
Water Electrolysis
- Water electrolysis methods include alkaline electrolysis and PEM membrane-based pure water electrolysis
- General hydrogen generators require drying or catalytic deoxygenation followed by frequent regeneration of drying agents
- Palladium membrane purifiers eliminate the need for drying agents and offer hydrogen purity levels of 6N–8N




